Vaccines developed against SARS-CoV-2 (COVID-19)
The virus that emerged in Wuhan, China, in December 2019 and caused acute respiratory syndrome; It was named SARS-CoV-2 due to its genetic and structural homology to the SARS-COV virus. Due to its global and rapid spread, it was officially declared a "pandemic" by the World Health Organization on March 11, 2020.
Signs of infection caused by the SARS-CoV-2 virus; From mildest to most severe, symptoms can range from a-symptomatic to minor flu-like symptoms, acute respiratory distress syndrome (ARDS), pneumonia and death.
Pandemic; Although it has been tried to be controlled by the use of masks, social distance, antiviral drugs, protease inhibitors and monoclonal antibodies, it has become necessary to develop a vaccine so that people can return to their normal social lives. Vaccines require a long time to develop, and unlike other drugs, they must be within safe limits because they are used in healthy individuals who are not sick.
According to World Health Organization data, as of August 11, 2020, 202 companies/research institutes are working on coronavirus vaccines. 29 of these research groups started clinical trials as of the same date.
Vaccines used in clinical trials are produced with 6 different technologies:
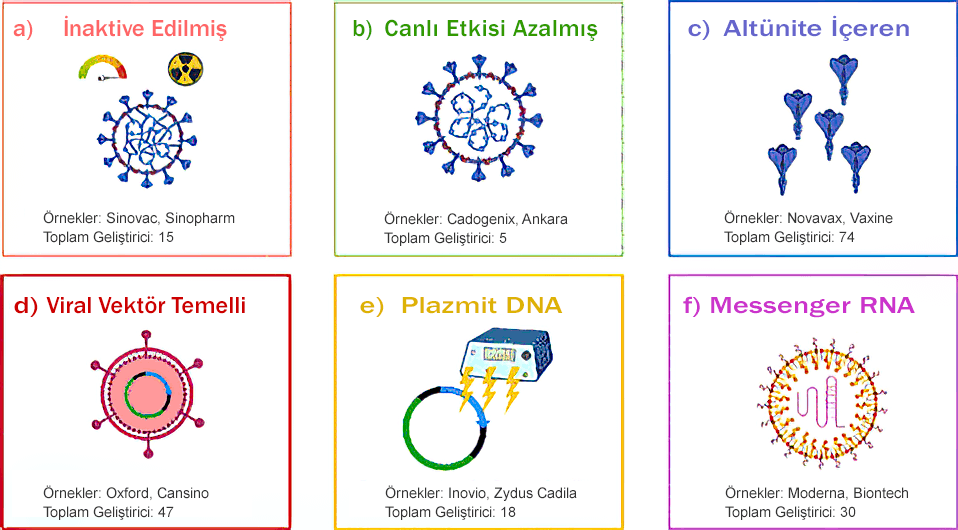
a) Inactivated vaccines: These are vaccines developed using viruses that have been inactivated by heat, radiation or the use of chemicals. In these vaccines, the genetic material of the virus is not replicated.
b) Live-attenuated vaccines: In these vaccines, the genetic material of the virus can replicate at a limited level.
c) Subunit-containing vaccines: This type of vaccines are vaccines that contain a subunit of the virus, such as the S protein (Spike - the protein found on the outer surface of the virus that allows it to bind to the receptor).
d) Viral vector-based vaccines: In these vaccines, the antigen is cloned into the viral vector. Commonly used viral vectors; lentivirus, adenovirus and adeno-associated virus (ADD). SARS-Cov vaccines use ADD as the viral vector.
e) Plasmid DNA vaccines: These vaccines do not use live viruses and are injected intradermally and taken into the cell by electroporation. Double-stranded DNA is more durable than virus proteins.
f) Messenger RNA vaccines: These are new generation vaccines. In these vaccines, the m-RNA molecule is encapsulated with nanoparticles. Cationic lipids are mostly used as nanoparticles.
Argument:
Around 100 vaccines have been produced around the world so far, and there is no one-size-fits-all solution. Clinical trials of many vaccines have not been completed yet.
Moderna and BioNtechPfizer; They are mRNA-based vaccines and contain the code required for the synthesis of the S (Spike) protein of the virus. S protein contains two subunits. While the S1 subunit enables the virus to bind to the ACE2 receptor on the surface of the cell, the S2 subunit is involved in the intracellular spread of the virus. In this vaccine, antibodies developed against the target S protein block the intracellular spread of the virus. Both vaccine manufacturers have disclosed safety and immunogenicity data. The vaccines were tested in healthy controls aged 18-55. There is no data on individuals over 55 years of age. The most important advantage of mRNA-based vaccines is that they can be produced quickly and mass-produced and they do not interact with host DNA. As for the disadvantages, the stability and half-life of m-RNA are very short. In terms of effectiveness, these types of vaccines have ten times less transfection efficiency than viral vector vaccines and have not been produced commercially before.
The “CoronaVac” vaccine, produced in cooperation with SinoVac and Wuhan Institute of Biological Products, contains inactive virus. The vaccine, which is currently undergoing phase 3 trials, was first administered to healthy controls aged 18-59, and individuals over the age of 60 were included in the phase 3 trials. In this vaccine, the virus inactivated by formalin is used together with adjuvant material to ensure stabilization. It is known that the adjuvant substance causes allergic reactions in some individuals.
The vaccines produced by Oxford/Astrazeneca and CanSino are viral-vector-based vaccines. Since the viral vector mimics the viral disease in this type of vaccines, a stronger cellular immune response occurs compared to recombinant vaccines. The advantage of this type of vaccines is that they are easy to produce and are chemically stable. As for the disadvantage; A special device is needed for intradermal electroporation.
Although all vaccine strategies have some advantages and disadvantages, vaccination will be an effective way to cope with the global pandemic that surrounds the whole world. However, it is not known how much the effectiveness of the vaccines will change depending on the mutations that occur on the virus.
REFERENCES:
1- The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation
Jieliang Wang,1 Ying Peng,2 Haiyue Xu,1 Zhengrong Cui,1 and Robert O. Williams , IIIcorresponding author1; AAPS PharmSciTech. 2020 Aug; 21(6): 225.Published online 2020 Aug 5. doi: 10.1208/s12249-020-01744-7
2- A Review of the Progress and Challenges of Developing a Vaccine for COVID-19
Omna Sharma,1,* Ali A. Sultan,2 Hong Ding,3 and Chris R. Triggle3, Front Immunol. 2020; 11:585354.
Published online 2020 Oct 14. doi: 10.3389/fimmu.2020.585354
3- COVID-19 Vaccine Frontrunners and Their Nanotechnology Design
Young Hun Chung 1, Veronique Beiss 2, Steven N Fiering 3 4, Nicole F Steinmetz; ACS Nano
2020 Oct 27;14(10):12522-12537. doi: 10.1021/acsnano.0c07197. Epub 2020 Oct 9. doi: 10.3389/fimmu.2020.585354
4- Progress and Pitfalls in the Quest for Effective SARS-CoV-2 (COVID-19) Vaccines
Katie L Flanagan 1 2 3 4, Emma Best 5 6, Nigel W Crawford 7 8, Michelle Giles 9 10, Archana Koirala 11 12 13, Kristine Macartney 11 12, Fiona Russell 7 8, Benjamin W Teh 14 15, Sophie Ch Wen
Review Front Immunol
2020 Oct 2;11:579250. doi: 10.3389/fimmu.2020.579250. eCollection 2020. DOI: 10.3389/fimmu.2020.579250
Department of Genetics
Elite Research and Surgical Hospital
Contact Us For Appointment:
Telephone line: 0392 444 3548 (ELIT)
Contact Form: https://www.elitenicosia.com/iletisim/